Reactions That Store Carbon Underground Can Cause Cracking. That’s Good News.
A laboratory experiment found that as CO2 solidified, it caused the rock around it to crack. In real reservoirs, this process could open up space to pump in more CO2.
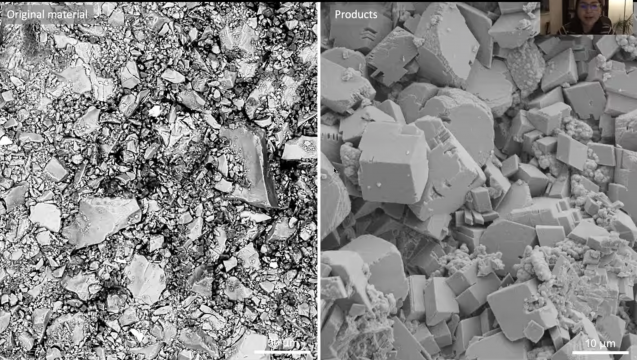
One promising way to remove carbon dioxide from the atmosphere is to pump it underground, where it can react with certain rocks that transform the gas into a solid mineral.
Scientists still have many questions to answer before this practice could be implemented on a large scale. One question is about what happens as the carbon mineralization process evolves — do the newly formed minerals clog the pores in the rock and prevent more CO2 from entering? Or do the additional minerals cause the surrounding rock to crack, opening up new areas where more CO2 can enter, react, and get stored?
New laboratory results, presented at the fall meeting of the American Geophysical Union on Monday, may have cracked the case. They suggest that while there is quite a bit of clogging over time, cracks also form, which can keep the reactions going in a self-sustaining loop. The research, which has not yet been published, was presented by lead author Catalina Sanchez-Roa, an associate research scientist at Columbia University’s Lamont-Doherty Earth Observatory.
“Carbon capture and storage is the only technology so far that can reduce atmospheric CO2 concentrations that are leading to climate change,” said Sanchez-Roa during a pre-recorded presentation. “We are interested in carbon mineralization because it is one of the most secure ways of storing carbon,” she added, and because it capitalizes on naturally occurring processes.
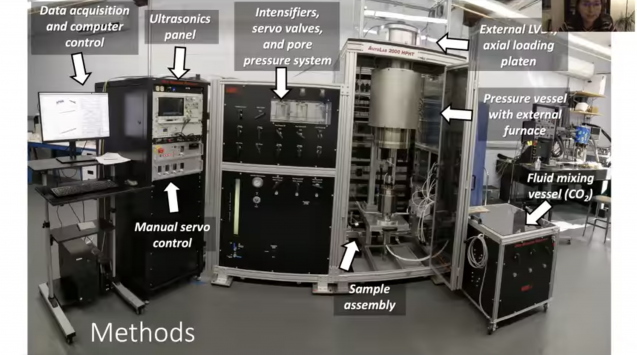
Sanchez-Roa and her colleagues started with a sample of dunite — a type of rock from the Earth’s mantle that can bond with CO2 to form solid carbonate minerals. The team ground up the dunite into a powder and pressed it together, forming a tube-shaped sample. Then they put the tube into a machine called a triaxial deformation apparatus, which simulates the temperature and pressure conditions that might be found underground in the real rock reservoirs that are being eyed for carbon storage. The machine also has a variety of sensors that measured how the properties of the rock material changed as the researchers repeatedly injected it with CO2 over a period of 35 days.
They found that the sample’s density increased over time, and its permeability decreased. This implies that some clogging took place while the carbon dioxide transformed into magnesite, quartz, silica, and elemental carbon.
The machine also measured several unexpected acoustic emissions which, combined with other measurements such as decreases in pore pressure and increases in volume, indicated that cracks were forming within the sample. The cracks seemed to help the permeability to remain low but steady, as opposed to continuously decreasing as it had earlier in the experiment.
The researchers note in their abstract that this is the first experimental evidence documenting cracking during the carbon mineralization process, and that the cracking helps to maintain permeability. They write: “These results confirm that the carbon mineralization process can be self-perpetuating through reaction-driven cracking (at least at the local scale), a process that is fundamental to upscaling engineered carbon mineralization as an efficient, and safe method for CO2 storage.”
Next, they hope to continue the experiments in rocks that are intact, and to explore which temperature and pressure conditions are best for encouraging cracking.
Sanchez-Roa’s co-authors include Ah-Hyung Alissa Park and Marc Spiegelman of Columbia University, and Jacob Tielke, Christine McCarthy, and Peter Kelemen of Columbia’s Lamont-Doherty Earth Observatory.
